GAINESVILLE, Fla. (October 31, 2023) – Exactech, a developer and producer of innovative implants, instrumentation, and smart technologies for joint replacement surgery, announced the successful first surgery using its new, advanced Activit-E™ polyethylene for the Truliant ® knee replacement system.
Hany Bedair, M.D., performed the procedure at Massachusetts General Hospital (MGH) in Boston, where the material was developed by renowned polyethylene expert Orhun Muratoglu, Ph.D., director of the Harris Orthopaedic Laboratory at MGH. Dr. Muratoglu was also in attendance for the knee replacement case.
“It is a profound moment when you have the inventor who developed our advanced polyethylene in the case, performed at the hospital where it was developed,” said Adam Hayden, CMO and SVP, Large Joints Business Unit at Exactech. “Exactech is extremely proud to offer this next generation of highly crosslinked polyethylene with vitamin E antioxidant.”
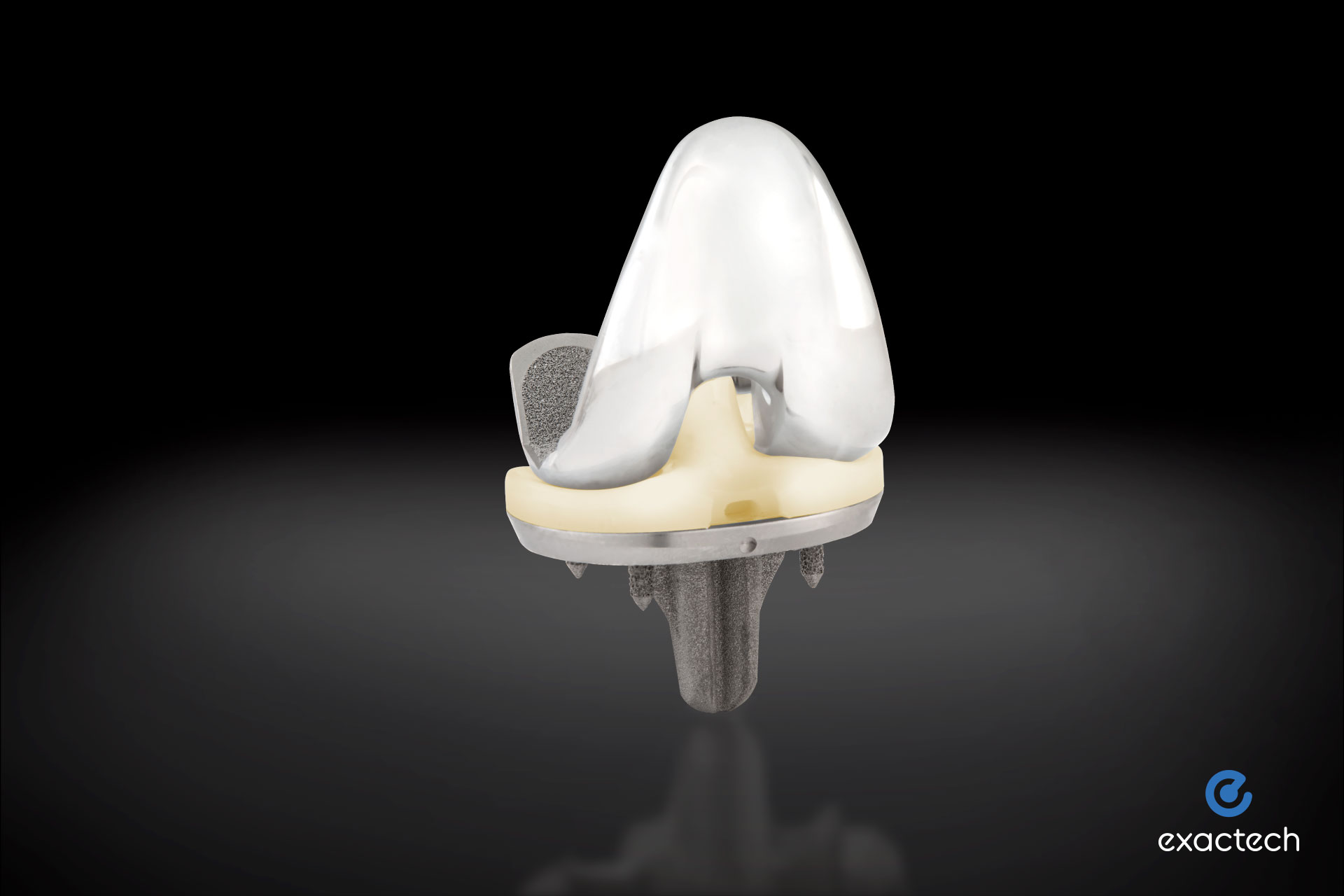
Activit-E offers an optimized balance of material strength and toughness through chemically crosslinked polyethylene, while eliminating the need for gamma irradiation technology used in previous generations. It allows for active oxidative resistance and a long-term, high-performance polyethylene bearing, ultimately providing greater strength and active stabilization.
...With this advancement, we anticipate better outcomes compared to knees with traditional, highly crosslinked polyethylene...
This new generation polyethylene is the latest, first-of-a-kind innovation by Dr. Muratoglu and his team, including Ebru Oral, Ph.D., director of biomaterials research. Muratoglu invented the first crosslinked polyethylene, and the first of multiple generations of vitamin E, antioxidant polyethylene for leading orthopaedic companies.
“It is exciting to perform the first surgical procedure with an implant material that was both conceived and developed within our hospital,” said Dr. Bedair. “With this advancement, we anticipate better outcomes compared to knees with traditional, highly crosslinked polyethylene.”
Activit-E was recently 510(k) cleared by the Food & Drug Administration (FDA) for Exactech knee and ankle replacements. Activit-E will be showcased at the 2023 AAHKS Annual Meeting in Dallas, Texas, Nov. 2-5.
References:
Knight, J, Rodgers III, William P, Freedman, Jordan. “Blended and Warm Irradiated vs. Infused: Mechanical Properties of Vitamin E Crosslinked UHMWPEs.” ORS 2017 Annual Meeting. Poster No. 2034.
Muratoglu, O, Asik, M, Nepple, C, Wannomae, K, Micheli, B, Connolly, R, Oral, E. “Di‐cumyl peroxide cross‐linked UHMWPE/vitamin‐E blend for total joint arthroplasty implants.” Journal of Orthopaedic Research. DOI: 10.1002/jor.25679.
About Exactech
Exactech is a global medical device company that develops and markets orthopaedic implant devices, related surgical instruments and the Active Intelligence® platform of smart technologies to hospitals and physicians. Headquartered in Gainesville, Fla., Exactech markets its products in the United States, in addition to more than 30 markets in Europe, Latin America, Asia and the Pacific. Visit www.exac.com for more information and connect with us on LinkedIn, VuMedi, YouTube, Twitter and Instagram. With Exactech by your side, you’ve got EXACTLY what you need.
Media Contact:
Morgan Lee
Marketing Communications Senior Manager
Morgan.lee@exac.com