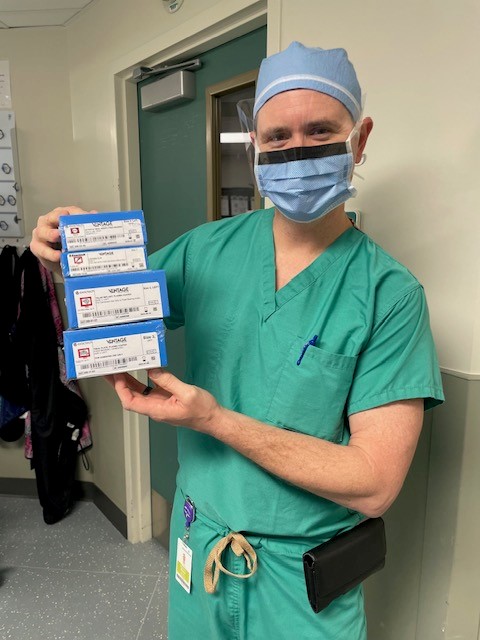

GAINESVILLE, Fla. (Feb. 29, 2024) – Exactech Quality Control Manager Wesley Garrett experienced a full circle moment in both his professional and personal life when his father received an Activit-E™ polyethylene insert after following the company’s polyethylene initiatives for six years.
In 2018, Garrett was hired as an intern to perform wear-testing for the company’s hip product line. Specifically, he tested results between vitamin E-infused polyethylene and standard polyethylene.
“I witnessed firsthand the wear-resistance improvements1 that come with the vitamin E infusion,” Garrett said. “Since then, I’ve anticipated the expansion of vitamin E poly to our other product lines and always thought to myself that if a family member or I needed a joint replacement, I would hope vitamin E poly would be an option.”
Serendipitously, last year Exactech announced Activit-E for the Vantage® Total Ankle System and his father, John Wesley Garrett. Sr., of Titusville, Fla., needed an ankle replacement.
John Wesley Garrett, Sr., a systems safety engineer at Kennedy Space Center for more than 35 years, experienced mobility difficulties at both work and home.
“My ankle injury contributed to a more sedentary lifestyle over the years. The injury made it difficult to perform, without pain, some duties in my professional career and limited physical activity with my family,” Garrett Sr. said. “I sat on the ‘sidelines’ many times during family activities while on vacation and other outings.”
...Activit-E, with its improved polyethylene wear properties, coupled with the Vantage Total Ankle System, are a winning combination...
Garrett Sr.’s ankle replacement surgery was performed earlier this month by Raymond Rowan, DPM, of Stellar Foot and Ankle in New Smyrna Beach, Fla. In addition, first surgeries for Activit-E were performed by various surgeons across the U.S., including Oliver Schipper, M.D., of the Anderson Orthopaedic Clinic.
“With any surgery, you want to use products and technologies that have the opportunity to enhance patient outcomes. Activit-E, with its improved polyethylene wear properties, coupled with the Vantage Total Ankle System, are a winning combination,” said Dr. Schipper.
As Garrett Sr. recuperates from surgery, he has high hopes for what the future holds.
“I am hoping this surgery will motivate me to become more active. I’m looking forward to a pain-free active retirement with my wife and friends, including travel to other countries. I’m also looking forward to participating in more family centered activities, including hunting, boating, hiking and cruising,” he said.
Son Wesley also has high hopes for the future and a renewed perspective toward his work.
“I feel a new sense of pride in our company and my work as a Quality Assurance professional as I get to be part of both the manufacturing production and the patient sides of the story. It’s really the moment where rubber meets the road for me on whether all that we say we do as a company and as professionals in the medical device industry makes the difference we say it does,” Garrett said. “So, in a way I feel both really nervous and excited to see our work on display in my family, and I feel a deep sense of gratitude to the many people from design all the way through operations and quality inspection who have worked diligently to produce our products.”
Activit-E is a next-generation highly crosslinked polyethylene with vitamin E antioxidant stabilization and was developed by Orhun Muratoglu, Ph.D., director of the Harris Orthopaedic Laboratory at Massachusetts General Hospital in Boston, and his team, including Ebru Oral, Ph.D, director of Biomaterials Research. Its unique manufacturing process replaces gamma irradiation crosslinking with peroxide crosslinking and adds vitamin E to provide strength, flexibility, toughness, and oxidative stability.2-3
Currently, Activit-E for the Vantage Ankle is in pilot launch with plans to increase availability through the end of the year. Learn more about Exactech’s foot and ankle solutions at www.exac.com/foot-and-ankle/vantage-total-ankle-system/. Exactech also provides Activit-E for use with its Truliant ® Knee Replacement System.
References:
- Data on file at Exactech, Inc.
- Knight, J, Rodgers III, William P, Freedman, Jordan. “Blended and Warm Irradiated vs. Infused: Mechanical Properties of Vitamin E Crosslinked UHMWPEs.” ORS 2017 Annual Meeting. Poster No. 2034.
- Muratoglu, O, Asik, M, Nepple, C, Wannomae, K, Micheli, B, Connolly, R, Oral, E. “Di‐cumyl peroxide cross‐linked UHMWPE/vitamin‐E blend for total joint arthroplasty implants.” Journal of Orthopaedic Research. DOI: 10.1002/jor.25679.
About Exactech
Exactech is a global medical device company that develops and markets orthopaedic implant devices, related surgical instruments and the Active Intelligence® platform of smart technologies to hospitals and physicians. Headquartered in Gainesville, Fla., Exactech markets its products in the United States, in addition to more than 30 markets in Europe, Latin America, Asia and the Pacific. Visit www.exac.com for more information and connect with us on LinkedIn, VuMedi, YouTube, Twitter and Instagram. With Exactech by your side, you’ve got EXACTLY what you need.
Media Contacts:
Samantha DiVirgilio
Marketing Communications Senior Manager
Samantha.divirgilio@exac.com