Empowering you to be your best.

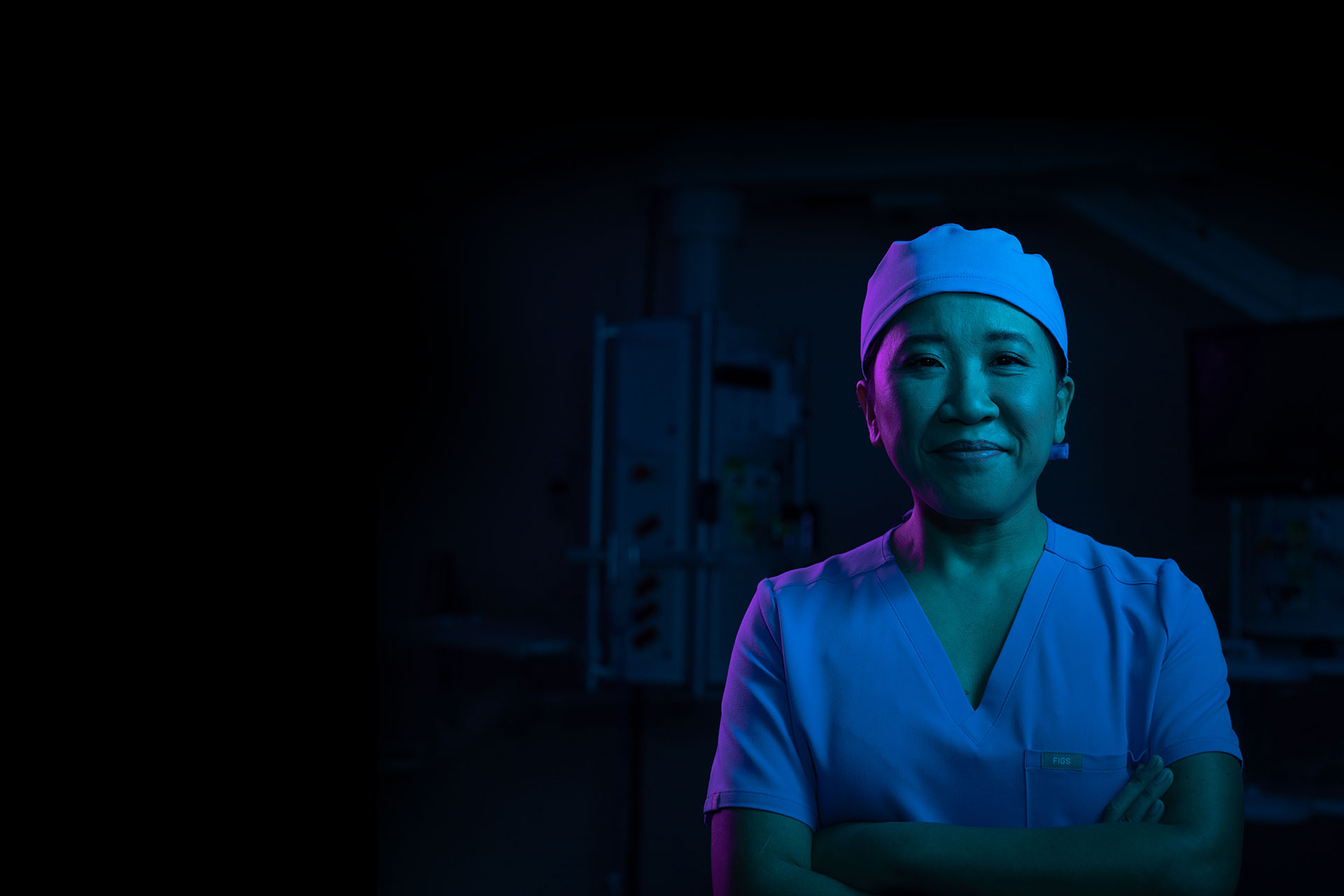
Supporting you in and out of the O.R.
Exactech AI is a dynamic ecosystem of enabling technologies and smart solutions that empowers surgeons with data-rich, low-cost solutions to help improve patient outcomes.
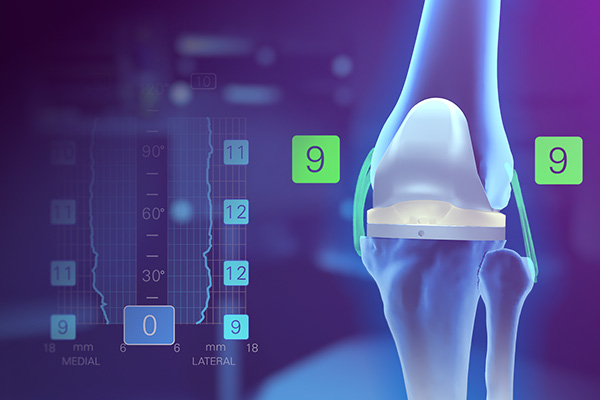
Knee System
Dynamic soft tissue analytics, pre-resection operative insights and full-range personalized planning.
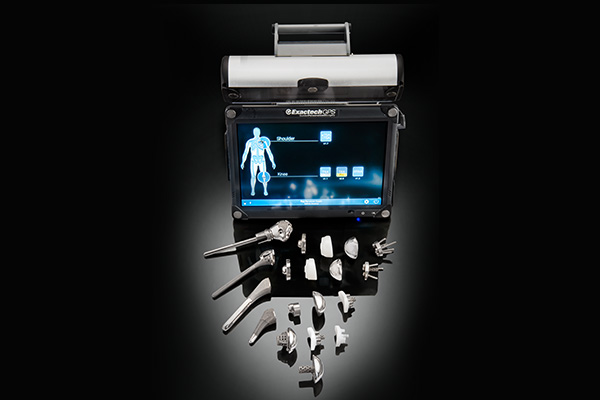
Shoulder System
Comprehensive range of innovative implants for both straightforward and challenging cases

Hip System
Extensive research review to identify the best of the best in design and materials

Foot and Ankle System
From our biomechanics-driven ankle design to customizable plate and screw system to patient specific instrumentation, we’ve got you and your patients covered.

Supporting you in and out of the O.R.
Exactech AI is a dynamic ecosystem of enabling technologies and smart solutions that empowers surgeons with data-rich, low-cost solutions to help improve patient outcomes.

Knee System
From primary to revision, a knee system that embodies the power to achieve reproducible results in a streamlined procedure

Shoulder System
12 years of clinical use and 85 peer-reviewed studies

Hip System
Extensive research review to identify the best of the best in design and materials

Foot and Ankle System
From our biomechanics-driven ankle design to customizable plate and screw system to patient specific instrumentation, we’ve got you and your patients covered.
Medical Education
Surgeons will gain exposure to the latest advancements in arthroplasty through various trainings.