INTRODUCTION
As long as there have been bone voids or defects, there has been a desire to fill them. The description of filling defects with various substances has been mentioned in ancient Hindu, Egyptian and Greek texts. One of the earliest well-documented examples of filling bone defects was by Dutch surgeon Jobi Meekren in 1682. Meekren implanted dog bone into the skull defect of a soldier. However, the church intervened in what they perceived as an unholy process and forced Meekren to remove the dog bone under threat of excommunication. Needless to say, there were no long-term outcomes to be reported to the literature.
Bone grafting was developed by many other European physicians such as Ollier, Duhamel and Syme, but the modern practice of bone grafting was invented by Scottish doctor Macewen in 1880. Fast forwarding to 1961, Peltier described his numerous experiments with using Calcium Sulfate Hemihydrate (CSH) to fill bone defects.
InterSep® Calcium Sulfate bone void filler consists of a latest generation, fully-synthetic CSH. CSH is also known as Plaster of Paris or in natural form — the mineral Bassanite. When CSH is mixed with the liquid solution included in the bone void filler kit, it reverts back to gypsum, which is also known as Calcium Sulfate Dihydrate (CSD).
Historically, gypsum deposits were found in the large hill of the Montmartre district of Paris. This is why CSH is called Plaster of Paris. Heating gypsum (CSD) releases water in the form of steam and results in CSH. The ancient Egyptians used Plaster of Paris to seal joints of the pyramids, as well as make casts of human figures. The Parisians used their plaster to plaster walls for fire resistance starting in the 18th century. The Dutch surgeon Mathysen popularized the use of Plaster of Paris as an orthopaedic dressing in the mid-19th century (1851 to be precise with a plaster bandage). In 1892, German physician Dreesmann documented healing of six of eight bone fractures with a mixture of phenoland Plaster of Paris, in what is possibly the first documentation of the use of a Calcium Sulfate bone void filler.
Since the use of Calcium Sulfate for medical purposes predated the existence of the Food and Drug Administration (FDA), Calcium Sulfate salts were classified as a Class II special controls device in 1998. These guidelines concern the purity and consistency of the material, as well as regulatory parameters required to bring new Calcium Sulfate-based products to market.
The summary below shows the biocompatibility, bench-top performance and animal testing carried out on InterSep® for the regulatory submission to the FDA. The product passed the acceptance criteria for the following required tests.

BIOCOMPATIBILITY TESTING
Cytotoxicity MEM Elution Test: The MEM Elution Test is designed to determine the cytotoxicity of extractable substances. An extract of InterSep was added to cell monolayers and incubated. The cell monolayers were examined and neither cell lysis nor any intracytoplasmic granules were found. The device is considered to be non-cytotoxic.
Bacterial Mutagenicity Test – AMES Assay: The Salmonella Typhimurium Reverse Mutation (AMES) Test employs several stains of S. typhimurium, which requires the amino acid histidine for growth to detect point mutations. The InterSep extract tested against the five strains did not meet the criteria for a potential mutagen. The device is found to be non-mutagenic.
ISO Maximization Test for Delayed Hypersensitivity: ISO 10993-10:2010 was used to determine if InterSep would cause delayed dermal contact sensitization in a guinea pig maximization test. The study results showed that InterSep extracts showed no evidence of causing delayed dermal contact sensitization in the guinea pig. The device is considered to be non-sensitizing.
ISO Acute Systemic Toxicity Test: The purpose of the study was to determine whether leachables extracted from InterSep would cause acute systemic toxicity following single-dose systemic injection into mice. The study result showed that there was no mortality or evidence of systemic toxicity form the extracts. Each test article extract met the test requirements. The device is considered to be nontoxic systemically.
ISO Intracutaneous (Intradermal) Reactivity Test: The purpose of the study was to determine whether leachables extracted from InterSep would cause local dermal irritant effects following injection into rabbit skin. The study result showed that there was no evidence of significant irritation from the extracts injected intracutaneously into rabbits. The device is not considered to be an irritant.
Chromosome Aberration: This test determines if the device causes any structural chromosome aberrations in Chinese Hamster Ovary (CHO) cells. The test complies with OECD and ISO guidelines as an in vitro diagnostic for genotoxicity. Results indicate no aberration in chromosome structure following exposure to the device.
ISO Materials Mediated Rabbit Pyrogen: This test determines whether a saline extract of the device causes a pyrogenic response (fever) in rabbits. This test is in compliance with ISO 10993-11. All extracts tested negative. The material is non-pyrogenic.
ISO In Vivo Mouse Micronucleous Assay: This test determined if the device induces micronuclei formation in immature polychromatic erythrocytes present in the bone marrow of adult mice. The presence of polychromatic erythrocytes is an indication of a mutagenic substance leached from the device. The test complies with ISO 10993-3. All extracts tested negative indicating the device is non-mutagenic.
InterSep bone graft material passed the requirements of all tests. It can be concluded that the product is biocompatible and non-toxic.
BENCH TOP PERFORMANCE TESTING
The manufacturer completed performance tests that simulated the intended physiological environment as outlined in FDA’s Class II Special Controls Guidance Document: Resorbable Calcium Salt Bone Void Filler Devices (2003). This section summarizes InterSep and the predicate devices’ performance test results.
The critical specifications of InterSep were compared to the predicate device. These analyses consisted of:
- Chemistry
- Crystallinity
- Physical form
- Porosity
- Dissolution/solubility
- pH
- Working time
- Setting time
- Dimensional stability
- Setting reaction temperature
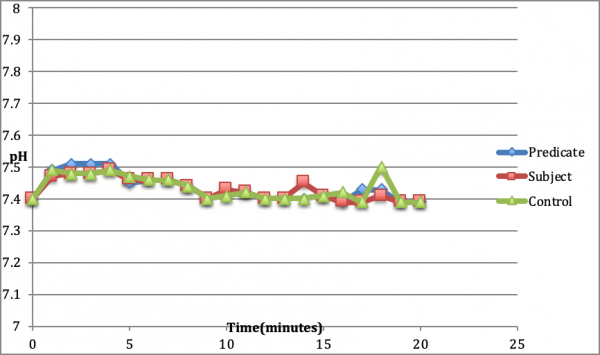
pH Testing: This test compared pH changes in surrounding simulated body fluids (Phosphate Buffered Saline (PBS)) of the predicate and subject test devices while the devices cured in vitro (Figure 1). The pH of the surrounding PBS for both devices were within the physiological pH of ~7.5.
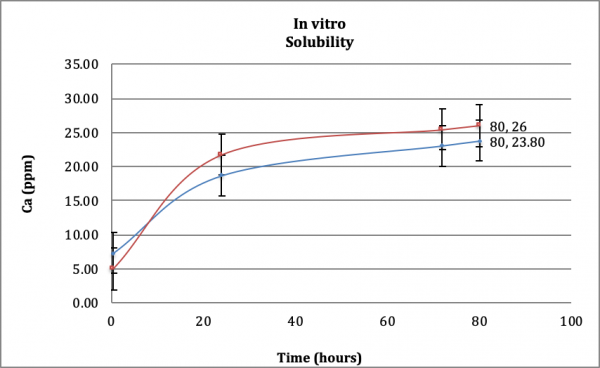
Dissolution/Solubility Testing – Solution Ionic Calcium Measurement by ICP-MS: Solubility of predicate and subject InterSep test devices were analyzed over 80 hours while immersed in Dulbecco’s PBS and maintained at physiological conditions (pH=7.4, 37° C) (Figure 2). The test purpose was to evaluate the in vitro dissolution behavior of the InterSep device compared to the predicate device. The calcium ion concentration at 80 hours for the test device was 23.8 ppm, and at the same time point, the predicate device was 26 ppm. The tested devices’ differences for the in vitro solubility test of the two minerals were negligible and were within experimental error (within 95% confidence interval). Over the course of the study, both products exhibited sparingly soluble calcium release.
The XRD results presented in Figure 2 displays the diffraction pattern for DB CSD.
Working Time: The test purpose was to examine the inter-operative handling properties of InterSep to ensure that the material has sufficient working time during surgical implantation in bone defects and/or voids (Figure 3).
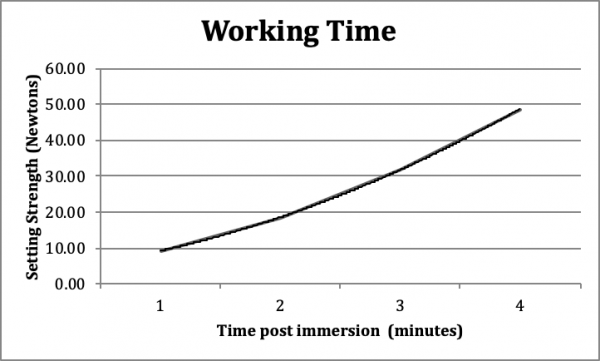
The working time of InterSep at 32° C was determined per the modification of the setting test (ASTM C403/C403M-99). The data showed an initiation set times of 3 minutes to attain a recordable load greater than 25 Newton when the curing temperature was 32° C. This indicated the maximum working time allowed before the material begins to harden in vivo was 3 minutes post-implantation. This will provide an ample opportunity for surgical adjustment, if needed.
Setting Time: The test purpose was to examine the setting properties of InterSep to ensure the bone void filler would set sufficiently hard in vitro and within a clinically relevant time under physiologic pH and temperature conditions (Figure 4). This test was a modification of the standard setting test described in ASTM C403/C403M-99 in which the load required to drive needles at a prescribed distance into concrete or a similar setting material was measured.
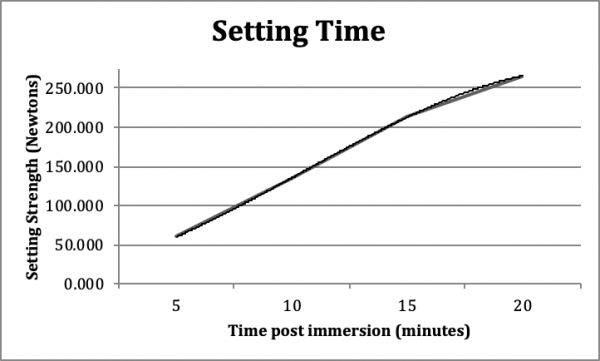
After immersion of 10 minutes in physiological conditions of temperature and pH, InterSep reached adequate strength presented in Appendix J. Data analysis yielded a set time of ~10 minutes to reach a load greater than 135 Newtons (N).
Dimensional Stability: Dimensional stability testing measured the volume change following incubation and setting at physiologic pH and temperature. InterSep hardened at 30 minutes in a contained volume with no physical shape change at 24 hours. Therefore, no change in physical shape would be expected following implantation in vivo.
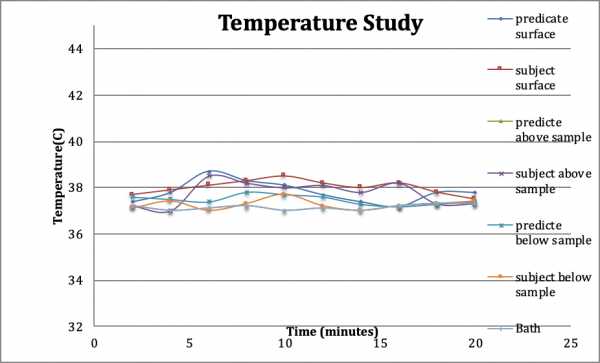
Setting Reaction Temperature: Some orthopaedic cement devices undergo an exothermic setting reaction that is of interest due to its biologic consequence. Both InterSep and the predicate devices had undergone hydration and setting via an isothermic reaction. The data indicated that the setting reactions of both tested devices did not significantly change the temperature of fluids within the setting material immediate vicinity (Figure 5). Temperature fluctuation was minimized and expected to ensure tissue compatibility. The results demonstrated that InterSep remained within the physiologic temperature range and is expected to have no adverse biologic consequence.
Chemical Analysis: Chemical and microstructural analysis per the FTIR, XRD, SEM and Porosimetry allowed a detailed composition and microstructure description of InterSep and predicate devices and to predict similarities in in vivo performance. All tests were performed on predicate and InterSep devices to establish substantial equivalence:
|
Properties Analyzed |
Technique and Tool |
|
Chemistry |
Fourier Transform Infrared Spectroscopy (FTIR) and X-ray Diffraction (XRD) |
|
Crystallinity |
X-ray Diffraction (XRD) |
|
Physical Form and Microstructure |
Scanning Electron Microscopy (SEM) |
|
Porosity |
Mercury Intrusion Porosimetry |
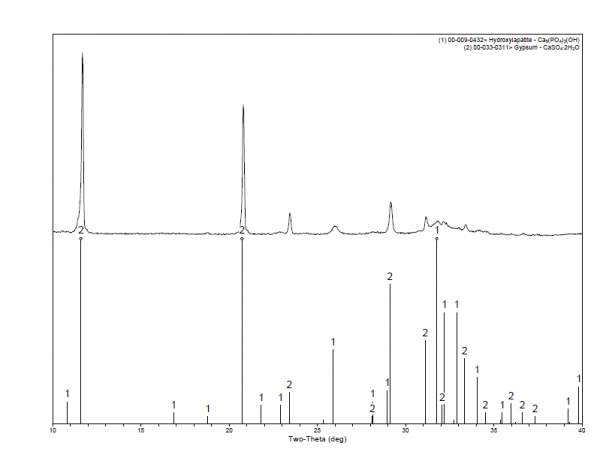
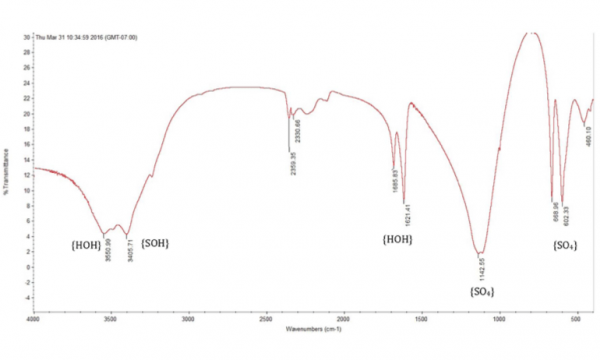
XRD and FTIR analyses confirmed that both predicate and subject devices were composed of CSD with no other phases detected (Figures 6 and 7, respectively). SEM and Porosimetry data indicated both tested devices resulted in the formation of intermingling, interlocking, nano-sized crystals of CSD after hydration and curing in vitro. Bulk density, pore diameter and total porous volume measured by mercury intrusion porosimetry showed that both subject and predicate devices were nano/micro porous materials with similar bulk densities.
Elemental Analysis: Heavy metal/trace elemental analysis was performed using inductively-coupled plasma mass spectroscopy (ICP-MS). ASTM standard F1185-03 (2009) suggests a limit for heavy metals/trace elements and is used as a reference. InterSep (DB CSD) contained substantially lower heavy metal/trace elements than limits described in the ASTM Standard with no heavy metal/trace elements present above 1 ppm (Table 1).
|
Element |
ASTM F1185-03 |
DB CSD |
|
Pb- Lead |
50 |
0.08 |
|
Hg-Mercury |
5 |
ND* |
|
As-Arsenic |
3 |
ND |
|
Cd-Cadmium |
5 |
0.012 |
*ND –Not Detected
ANIMAL TESTING
Ovine Cancellous Bone Defect
As described in the Class II Special Controls Guidance Document: Resorbable Calcium Salt Bone Void Filler Device (2003), Pacific Bioceramics, demonstrated that the subject device had the same critical specifications (i.e., chemistry, crystallinity, physical form, porosity, dissolution/solubility) and the same intended use as the predicate device.
A large animal critical-sized ovine defect model evaluated the biocompatibility, tissue reaction, implant resorption, bone formation and surgical handling properties of InterSep following implantation in the femoral and tibial metaphases. This ovine model compared the subject device (InterSep) with the predicate device (Stimulan®). Both devices are comprised of a calcium salt cementitious phase that is combined at time of use with an aqueous solution.
After implantation into sheep cancellous bone sites, histological data did not exhibit any adverse tissue response from either device. Both materials were biocompatible with normal bone remodeling that occurred around the periphery of the cylindrical implant area. There was no visible inflammatory reaction associated with the implanted devices, and no macrophages or giant cells were observed within the implant. Tissue fibrosis was not observed within the implanted regions. Histomorphometric analysis showed complete device resorption and similar bony ingrowth rates between subject and predicate devices over the implantation period of three months.
The InterSep device maintained the reported safety profile of the predicate device with no remarkable safety issues
DISCUSSION AND CONCLUSION
As described in the Class II Special Controls Guidance Document: Resorbable Calcium Salt Bone Void Filler Device (2003), Pacific Bioceramics, demonstrated that the subject device had the same critical specifications (i.e., chemistry, crystallinity, physical form, porosity, dissolution/solubility) and the same intended use as the predicate device.
A large animal critical-sized ovine defect model evaluated the biocompatibility, tissue reaction, implant resorption, bone formation and surgical handling properties of InterSep following implantation in the femoral and tibial metaphases. This ovine model compared the subject device (InterSep) with the predicate device (Stimulan®). Both devices are comprised of a calcium salt cementitious phase that is combined at time of use with an aqueous solution.
After implantation into sheep cancellous bone sites, histological data did not exhibit any adverse tissue response from either device. Both materials were biocompatible with normal bone remodeling that occurred around the periphery of the cylindrical implant area. There was no visible inflammatory reaction associated with the implanted devices, and no macrophages or giant cells were observed within the implant. Tissue fibrosis was not observed within the implanted regions. Histomorphometric analysis showed complete device resorption and similar bony ingrowth rates between subject and predicate devices over the implantation period of three months.
The InterSep device maintained the reported safety profile of the predicate device with no remarkable safety issues
InterSep® is manufactured by Pacific Bioceramics and distributed by Exactech, Inc.